Orbital Toys!
Atoms are not flat
While the Journey to Neon games use flat symbols to represent atoms and elements, the protons and neutrons are thought to be clustered together in the center of an atom as a sphere. The electrons spend their time in neighborhoods around an atom referred to as orbitals. Some orbitals are thought to be spherical, while others are thought to have a variety of interesting shapes. These shapes represent a cloud inside of which up to two electrons can be found anywhere inside. In the first ten elements, there are said to be two types of orbitals: s orbitals are considered spherical clouds; p orbitals clouds are like two teardrops connected by their pointed ends, called a dumbbell shape, the center of which is at the center of the atom.
Here are the five orbital toys which represent the first ten elements:

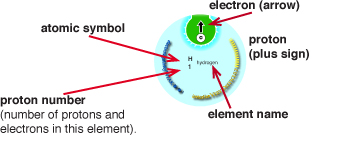
The green sphere on the left represents the first orbital around the atom called the 1s orbital. Hydrogen or helium would be represented by this green sphere if the games were three dimensional. In the cards and games, the 1s orbital is represented by a green circle, like in the Electronimoes cards:
Get a feel for orbitals.
Make
a neon ornament out of felting wool:
The two electrons in the 1s orbital are said to be at the same energy level. Once this orbital is full, the next eight electrons are found further from the center of the atom in what is sometimes referred to as the second shell around the atom. In this shell, there are four more orbital homes for electrons. There is one s orbital and three p orbitals. Since these orbitals are in the second shell, they start with the number 2. The RIGHT most orbital toy has a blue sphere, which represents the 2s orbital. The 2p orbitals are magenta, yellow and red. These three orbitals are called the 2px, 2py and 2pz orbitals, as they are oriented in three different directions called x, y, and z.

Embedded in this blue sphere are three p orbitals. These are the dumbbell shapes, and are red, yellow, and magenta in the toys. If you could see these orbitals by splitting the toy in half through them, it would look something like this:
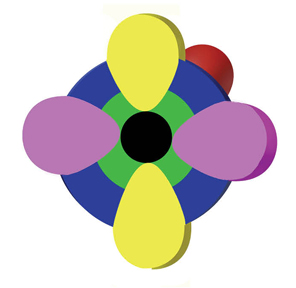
The green 1s orbital is included in this cross section. The black center represents the nucleus. The toys can be opened up to reveal the green 1s orbital inside, pictured next. Electron arrows may be velcroed on to the black dots; two in the green 1s orbital labeled "1" and "2" in red and white: two more can be fixed next to the "3" and "4" in the blue 1s orbital. The remaining six electrons in the second shell go on the velcro dots on each p orbital:

The 2p orbitals (magenta, red and yellow) are considered lower in energy than the 2s orbitals. What about the other three toys? They represent atoms with hybrid orbitals. Neon is considered unhybridized, but in some elements the orbitals around an atom can merge together to make hybrid orbitals. Hybrid orbitals have an energy somewhere between that of either s or p in the first ten elements. These are painted according to which orbitals combined to make them. Here is a video that helps explain hybrid orbitals while guiding you through a craft project where you can make your own hybrid orbital element!
Making an sp3 hybrid orbital ornament out of felting wool